Background
Cold agglutinin disease (CAD) is a rare autoimmune hemolytic anemia (AIHA). Rituximab is approved for treatment of B-cell lymphoma and severe rheumatoid arthritis and used off-label as a treatment for AIHA, including CAD. There is limited real-world evidence on patterns of rituximab use, safety, response rate and duration of response among patients with CAD.
Objectives
The key objectives were: 1) to characterize patients who initiated rituximab and examine their treatment patterns; 2) to describe the incidence of serious infections (SIs) prior and post rituximab treatment; 3) to assess the rate and duration of response to rituximab using hemolytic markers.
Methods
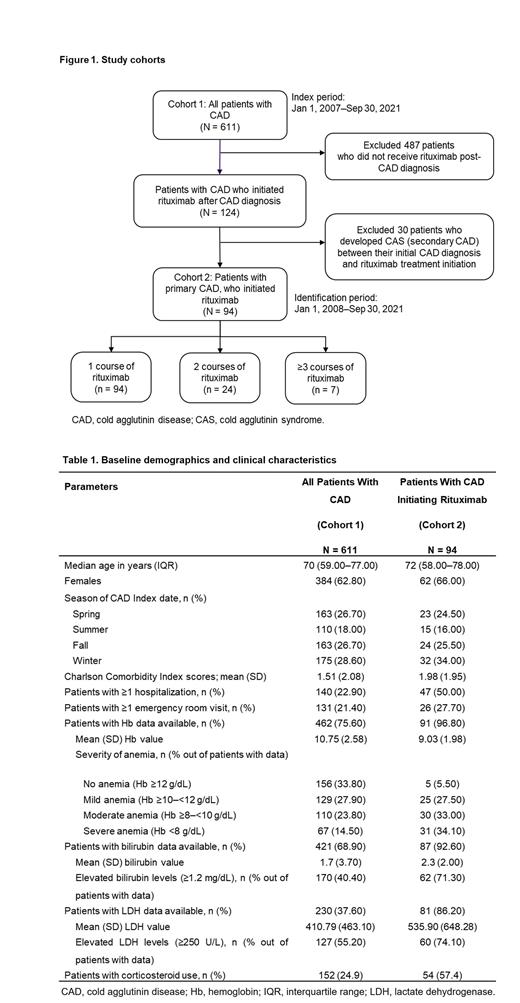
This retrospective, cohort study included adult (aged ≥18 years) patients with CAD from Optum's de-identified Market Clarity Data (2007-2021). Patients with CAD (Cohort 1) were indexed on their CAD diagnosis date. Cohort 1 patients who had ≥1 rituximab infusion (Cohort 2) were indexed on their 1 st rituximab infusion date ( Figure 1). Patients with cold agglutinin syndrome associated comorbidities on or before their index date were excluded. For each cohort, demographics, and clinical characteristics at index date, and for Cohort 2, clinical characteristics during 1 year before index date were described. Data for rituximab treatment patterns included number of courses, treatment type (monotherapy or combination therapy), complete or incomplete course, and the use of blood transfusion (BT) as rescue therapy. In Cohort 2, the incidence rates of SIs per 1000 patient-years before and after treatment (within 6 months of 1 st infusion) were assessed. Response to treatment was assessed using hemolytic markers (hemoglobin [Hb], bilirubin, and lactate dehydrogenase [LDH]). From baseline, a change of ≥2 g/dL was considered a Hb response, while a decrease of 50% was assessed as proxy for a bilirubin or LDH response. Relapse was defined as the reversal of biomarker levels below the minimum response threshold. Response duration was the period from the date of response to that of relapse.
Results
A total of 611 CAD patients were identified (Cohort 1); 94 of them received rituximab (Cohort 2). Median time from CAD diagnosis to rituximab initiation was 43.5 (Interquartile range [IQR]: 13.0-308.3) days.
Overall, Cohort 2 had a more severe disease status at treatment initiation than all patients with CAD (Cohort 1) at diagnosis (higher comorbidity scores, rates of health care resource utilization, lower Hb, and more severely elevated hemolytic markers; Table 1). In Cohort 2, the mean (SD) number of rituximab courses per patient was 1.45 (1.16); 24 (25.5%) of patients had ≥1 incomplete course; 37 (39.4%) required BT rescue therapy, and the median time from rituximab initiation to 1 st BT was 31 (IQR: 1.5-110.5) days. Combination therapy was rarely used (3/94; 0.03%); only 1 patient completed.
The incidence rate (95% CI) of SIs (per 1000 patient-years) post CAD diagnosis in Cohort 2 was ~3 times higher in the 4 to 6 months after rituximab initiation (637.1 [242.2-1032.0]) than before (245.4 [125.1-365.6]). Proportion of corticosteroid use was the same during 1 year prior to rituximab initiation and concomitantly with rituximab (54/94; 57.4%, both).
For patients in Cohort 2 with available data, 69.5% (41/59) had a Hb response, 59.6% (28/47) had a bilirubin response, and 31.3% (10/32) had a LDH response to the 1 st course of rituximab (median [IQR] time to response: 48.0 [33.5-85.0], 68.0 [33.3-199.8], and 67.5 [45.3-211.3] days, respectively). Among patients who responded to the 1 st course, Hb relapse occurred in 68.3% (28/41), bilirubin relapse in 53.6% (15/28), and LDH relapse in 80.0% (8/10) of patients (median [IQR] time from response to relapse: 44.0 [13.3-90.8], 98.00 [29.0-257.0], and 93.00 [21.3-161.8] days, respectively).
Conclusions
The findings highlight outcomes of rituximab use for treatment of CAD in a real-world setting. Patients who initiated rituximab had more severe disease compared to all patients with CAD. Rituximab was predominantly given as monotherapy, with an average of 1.45 courses per patient. About 25% of patients had ≥1 incomplete rituximab course, and rescue therapy with BTs was often required. Patients receiving rituximab experienced 3 times more SIs after initiating rituximab than before treatment. Although responses to rituximab were commonly observed, duration of response was short, and the rate of relapse was high.
OffLabel Disclosure:
Piatek:Apellis: Membership on an entity's Board of Directors or advisory committees; Argenx: Research Funding; Celgene: Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Annexon Biosciences: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees, Research Funding; Osotec: Research Funding; Incyte: Research Funding; Alexion, AstraZeneca Rare Disease: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding. Murakhovskaya:Sanofi, Novartis, Alexion, Jansen, Rigel, and Incyte: Research Funding; Alexion, Apellis, Janssen, Novartis, Rigel, and Sanofi: Speakers Bureau; Alexion, Apellis, Janssen, Novartis, Rigel, and Sanofi: Consultancy. Karaouni:Sanofi: Consultancy. Miles:Sanofi: Current Employment; Sanofi: Current equity holder in publicly-traded company. Heller:Sanofi: Current Employment; Aetion: Ended employment in the past 24 months. Lucia:Aetion: Current Employment. Patel:Sanofi: Current Employment. Tyma:Sanofi: Current Employment. Yoo:Sanofi: Current Employment, Current equity holder in publicly-traded company. Gertz:Ionis/Akcea, Prothena, Sanofi, Janssen, Aptitude Healthgrants, Ashfield, Physicians Education Resource, Research to Practice, Johnson & Johnson, and Celgene: Consultancy; i3HEalth: Other: For development of educational material; AbbVie: Other: Data Safety Monitoring board; Juno Pharmaceutics, and Sorrento Therapeutics: Other: Meetings.
Rituximab is approved for treatment of B-cell lymphoma and severe rheumatoid arthritis and used off-label as a treatment for autoimmune hemolytic anemia, including cold agglutinin disease.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal